
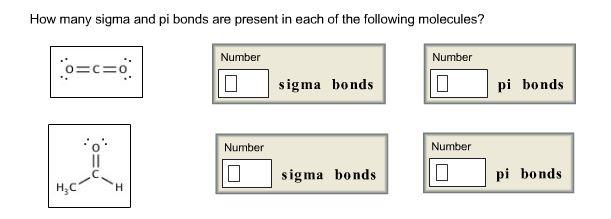
In such type of overlapping occur along internuclear axis when one half filled orbital will undergoes head-on overlapping. Such condition can be represented as follows: In such type of overlapping occur along internuclear axis when one half-filled p orbital overlaps with one half fille s orbital, and forms the covalent bond. In such type of overlapping one ‘s orbital will participate along internuclear axis which means head-on overlapping. Singly bonded electrons are formed as according to the combination of atomic orbitals as follows: S-S overlapping-: The electrons which are participating in bond formation are termed as sigma electrons. Types of Overlapping:ĭirect overlapping can be seen in sigma bonds. It is found that sigma bonds are stronger in comparison with pi bonds. In sigma bond, the orbitals are as similar as according to “s” where as in pi bond the orbital is similar with p orbitals. The sigma and pi bonds both are used to predict the molecules behaviour as according to molecular orbital theory. The names are derived from the Greek letters. The bonds are formed by electron transfer of specific wavelength of 2s electron to 2pz.Here one 2s orbital will combine with one 2p orbital to form sigma bond and sp hybridization whereas the remaining 2py and 2pz forms the pi bond. Examples of Pi bonds:Įthyne bonding is the ones such example of pi bonding, which is singly bonded with one hydrogen atom and triple bonds are present mong carbon atoms. In case of triple bonds one sigma and two pi bonds are present. In general, when single bond is present in between the two atoms it is sigma bond where as double bonds are comprising of one sigma bond and one pi bond. Therefore, it is noted that the two bonds are formed sigma bond and pi bond in covalent molecule of ethene. Pi bond can be represented as π.In pi bond the electron density is concentrated on both above and below of the plane of nuclei of bonding atoms. As according to Lewis dot structure, double bond which is formed in between molecule can be represented by single dash. The pi bond is the second bond that is formed in between C-C atom and are elongated on both above and below the plane of the molecule. This can be seen in the picture depicted below where hydrogen bonded singly as sigma bond. Sigma bond can be seen in the formation of hydrogen molecule. Such type of overlapping is called axial overlapping. Sigma bonds are formed as end to end which means the overlapping is done coaxially. Six sigma bonds are formed in total out of which three are bonded with carbon atoms in the molecule. The bond formed by the cation of end to end overlap of sigma bonds, where the concentrated density of electrons was found in nuclei of atom and the bonding atoms.
The single bond formed is termed as Sigma Bond. So, in above discussion it is mandatory to distinguish the two covalent bond formed in ethene.


 0 kommentar(er)
0 kommentar(er)
